
Fluorination Strategies


Photocatalytic Fluorination:
Over the past several years we have reported several methods for introducing fluorine into complex molecules including a direct fluorination of unactivated C-H bonds. This reaction utilizes the decatungstate anion and N-fluorobenzenesulfonimide (NFSI) for photocatalytic hydrogen atom abstraction and fluorine atom transfer. The decatungstate/NFSI fluorination displays excellent functional group tolerance including the ability to cleanly and selectively fluorinate unprotected amino acids and leucine residues in unprotected peptides. Current work in this area involves exploring the scope and limitations of our fluorination reaction with a goal of assisting medicinal chemistry efforts.
Highlighted Works:
- Halperin, S.D., et al. 'A Convenient Photocatalytic Fluorination of Unactivated C-H Bonds' Angew. Chem. Int. Ed., 2014
- Nodwell, M.B., et al. 'Direct photocatalytic fluorination of benzylic C-H bonds with N-fluorobenzenesulfonimide' Chem. Comm., 2015
- Halperin, S.D., et al. 'Development of a Direct Photocatalytic C-H Fluorination for the Preparative Synthesis of Odanacatib' Org. Lett., 2015
- Meanwell, M., et al. 'Synthesis of Acyl Fluorides via Photocatalytic Fluorination of Aldehydic C-H Bonds' Chem. Comm., 2018
- Yuan, Z., et al. 'Electrostatic Effects Accelerate Decatungstate-catalyzed C-H Fluorination using [18]F- and [19F]NFSI in Small Molecules and Peptide Mimics' ACS Catalysis, 2019
Radiofluorination:
Positron emission tomography (PET) imaging is an imaging technique that allows functional measurement of biochemical and physiological processes in living tissue. Commonly used in oncology, PET relies on radiotracers - a ligand labeled with a positron-emitting isotope - whose distribution and concentration can be detected through isotopic decay. Of all the radionuclides used in PET, 18F is closest to being an ideal radionuclide (t1/2 = 110 min, good metabolic stability, small size), and is used in the most common PET radiotracer, 18F-fluorodeoxyglucose (18F-FDG). However, the introduction of 18F into sensitive organic frameworks remains a challenge, thus limiting the structures available to nuclear medicine clinics. The Britton group, in collaboration with TRIUMF - Canadas national particle accelerator facility - has been involved in the discovery of novel methods for 18F fluorination since 2014. One of these, the DT-PhotoFluor platform has been extensively used for the 18F fluorination of unactivated C-H bonds, such as the 3o position of leucine and leucine analogues. This platform has allowed us to generate novel oncological PET radiotracers which may be of use in imaging multiple myeloma - a disease that is not well served by current imaging technologies. Other radiofluorination reactions we have investigated include the 18F fluorination of thiocarbonyls and dithianes via Ag18F.
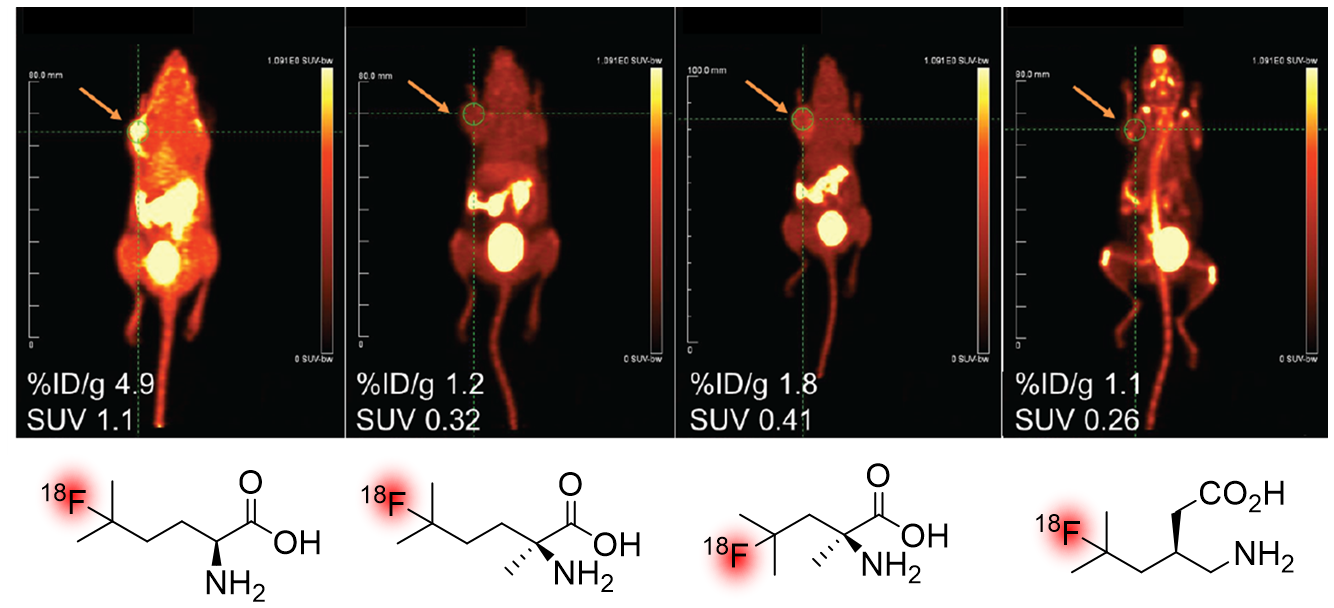


Highlighted Works:
- Nodwell, M.B., et al. '18F-Fluorination of Unactivated C-H Bonds in Branched Aliphatic Amino Acids: Direct Synthesis of Oncological Positron Emission Tomography Imaging Agents' JACS, 2017
- Yuan, Z., et al. 'Site-Selective, Late-Stage C-H 18F-Fluorination on Unprotected Peptides for Positron Emission Tomography Imaging' Angew. Chem. Int. Ed., 2018
- Nodwell, M.B., et al. '18F-branched chain amino acids: structure-activity relationships and PET imaging potential' J. Nucl. Med., 2019
- Newton, J., et al. 'A Convenient Synthesis of Difluoroalkyl Ethers from Thionoesters using Silver (I) Fluoride' Chem. Euro. J., 2019
- Yuan, Z., et al. 'Electrostatic Effects Accelerate Decatungstate-catalyzed C-H Fluorination using [18]F- and [19F]NFSI in Small Molecules and Peptide Mimics' ACS Catalysis, 2019
- Newton, J., et al. 'Rapid 18F- and 19F-Difluoromethylation through Desulfurative Fluorination of Transient N-, O-, and C-Linked Dithioles' Chem. Euro. J., 2022

Other Fluorination Strategies:
Our continuing interests in unique fluorination processes led to the discovery that thionoesters, aryl thionocarbonates, and dithianes undergo fluorodesulfuration using silver(I) fluoride, rapidly affording difluoroethers, difluorobenzodioxoles, and difluoromethyl compounds respectively. We have also synthesized and studied tertiary alkylammonium trifluoromethoxide salts as bench stable sources of trifluoromethoxide and developed a method for late-stage heterobenzylic mono and difluorination using N-fluorobenzenesulfonimide (NFSI).
Highlighted Works:
- Meanwell, M., et al. 'A Convenient Late-Stage Fluorination of Pyridylic C−H Bonds with N-Fluorobenzenesulfonimide' Angew. Chem. Int. Ed., 2016
- Meanwell, M. and Britton, R. 'Synthesis of Heterobenzylic Fluorides' Synthesis, 2018
- Meanwell, M., et al. 'Direct Heterobenzylic Fluorination, Difluorination and Trifluoromethylthiolation with Dibenzenesulfonamide Derivatives' Chem. Sci., 2018
- Newton, J., et al. 'A Convenient Synthesis of Difluoroalkyl Ethers from Thionoesters using Silver (I) Fluoride' Chem. Euro. J., 2019
- Newton, J., et al. 'Quaternary Ammonium Trifluoromethoxide Salts as Stable Sources of Nucleophilic OCF3' Org. Lett., 2020
- Newton, J., et al. 'Fluorodesulfurization of Thionobenzodioxoles with Silver (I) Fluoride' JOC, 2020
- Newton, J., et al. 'Rapid 18F- and 19F-Difluoromethylation through Desulfurative Fluorination of Transient N-, O-, and C-Linked Dithioles' Chem. Euro. J., 2022
